The Department of Antimetabolies of Nucleid Acid Components
The chemistry of nucleic acids is a traditional and continuously developing topic at the IOCB. In the 1960s, a department was formed around the group of scientists who studied the chemistry of nucleobases, nucleosides and oligonucleotides. Over the years, this department has made principal contributions to the development of the field. Outstanding achievements of the past encompass original studies on 6-azapyrimidines and 5-azapyrimidines and their nucleosides, which resulted in the discovery of potent antimetabolites (6-azauridine, 2′-deoxy-5-azacytidine), fundamental investigation on the mechanisms of nucleosidation reactions, sugar-modified nucleosides and the transformation of nucleoside molecules. In oligonucleotide chemistry, the methods of chemical and enzymatic oligoribonucleotide synthesis have been developed in this department. The research traditionally combines the development of novel biologically active compounds with the investigation of their metabolism and mode of action. It is based upon numerous joint investigations of different subjects in medicine: immunology, virology (studies on retroviruses, DNA viruses), antitumor and antileukemic studies etc., both domestically and abroad. The tissue culture laboratory run by the department performs the screening of the cytostatic activity in vitro.
The present research of the research group concerns mainly the design and synthesis of the analogues of nucleic acid components including modified nucleobases, nucleosides with modified sugar moiety and nucleotides with modified phosphomonoester linkage (acyclic nucleoside phosphonate analogues, ANP).
This new class of compounds was discovered at the IOCB by Prof. Antonín Holý in the early 1980s, with the aim of creating catabolically stable, isopolar and, possibly, isosteric, nucleotide analogues. ANP analogues exhibit a unique spectrum of antiviral and antitumor activity, and three compounds of the ANP group (cidofovir, adefovir, tenofovir) are active components of potent antivirals approved for therapeutic use in human medicine. HPMPC (cidofovir), PMEA (adefovir), and PMPA (tenofovir) have proven to be effective in vitro (cell culture systems) and in vivo (animal models and clinical studies) against a wide variety of DNA virus and retrovirus infections: cidofovir against herpesvirus (herpes-simplex virus types 1 and 2, the varicella-zoster virus, cytomegalovirus [CMV], the Epstein-Barr virus, and human herpesviruses), polyomavirus, papillomavirus, adenovirus, and poxvirus (variola virus, cowpox virus, vaccinia virus, molluscum contagiosum virus, and orf virus) infections; adefovir against herpesvirus, hepadnavirus (human hepatitis B virus) and retrovirus (human immunodeficiency virus types 1 [HIV-1] and 2 [HIV-2], simian immunodeficiency virus, and feline immunodeficiency virus) infections; and tenofovir against both hepadnavirus and retrovirus infections. Cidofovir (Vistide) has been officially approved for the treatment of CMV retinitis in AIDS patients, tenofovir disoproxil fumarate (Viread) has been approved for the treatment of HIV infections (i.e. AIDS), and adefovir dipivoxil (Hepsera) has been approved for the treatment of chronic hepatitis B.
DUVIRAGEL™
(antiherpeticum)
Active against fever blisters. It was manufactured by the Spofa company in the Czech Republic.
The Institute has established a long-term cooperation with the American biopharmaceutical company, Gilead Sciences, which deals with research, development and distribution of novel pharmaceuticals. The Institute and the company established a joint research center on July 13, 2006. The long-term collaboration based on the research of Prof. Antonín Holý has led to the development of a set of antiviral drugs, which are briefly listed below.
VISTIDE™
(cidofovir injection)
Treats opportunistic diseases accompanying AIDS (gastric ulcers, herpes zoster, smallpox, viral inflammation of the eyes, etc.).
VIREAD™
(tenofovir disoproxil fumarate)
An antiviral drug for AIDS treatment which helps block reverse transcriptase – a special protein that is needed for HIV to replicate. By interfering with the replication process, Viread as part of combination therapy helps lower the viral load. The active compound is tenofovir. This drug has been also approved for use in HBV infections.
HEPSERA™

(adefovir dipivoxil)
Active against hepatitis type B. It is based on adefovir and has practically no side effects.
TRUVADA™

(emtricitabine and tenofovir disoproxil fumarate)
For use in combination with other HIV medications to treat HIV infections in adults. Truvada combines two HIV medications, Emtriva (emtricitabine) and Viread (tenofovir), into one pill, which is taken once a day. It replaces 13 other tablets.
ATRIPLA™
(efavirenz, emtricitabine and tenofovir disoproxil fumarate)
The first-ever once-daily single tablet regimen for HIV intended as a stand-alone therapy. The product combines Sustiva (efavirenz) and Truvada (emtricitabine and tenofovir). It replaces the vast number of pills that patients formerly had to take.
Written by Dr. Martin Flegel
Since the early 1950s, the research of peptides at the Institute of Organic Chemistry and Biochemistry (IOCB) of the Czechoslovak Academy of Sciences has focused on the Neurohypofyseal hormones – their synthesis and biological effects. When Vincent du Vigneaud received his Nobel Prize in 1955 for his pioneering synthesis of Oxytocin and Vasopressin, the research in this field at the IOCB considerably progressed and almost reached the goal of being the first in the industrially applicable synthesis of Oxytocin.
In 1958, the industrial production of synthetic Oxytocin started at the Leciva-Pharmaceuticals company.
In about two years, Lysin-vasopressin (LVP) was put into production at the same pharmaceutical company.
Oxytocin was and still is used as the basic drug in gynecology during childbirth; Lysin-vasopressin was originally used in the treatment of diabetes insipidus, but its side effects (pressoric activity and myocardial toxicity) have prevented its broad utilization in this treatment. Nowadays, it is still used at low concentrations in dentistry (as an additive to local anesthesia).
The research which has continued at the IOCB’s peptide department and structure-activity studies have enabled the rational replacement of amino-acid residues in various positions of above-mentioned hormone structures and the preparation of new analogues of hormones without the undesirable effects.
Oxytocin could be replaced in obstetrics-gynecology applications by Metyloxytocin which was safer in the cases when protracted delivery took place; unfortunately, due to marketing problems, the production of this drug was rather low and stopped.
Another excellent OT analogue, Carbetocin, was synthesized later. Despite its very promising biological activity profile and at the same time enhanced stability, this peptide was used for many years as a veterinary drug only. In the last ten years have been exploited its very suitable and useful properties in human medicine.
Carbetocin have been registered by Ferring for human use as Duratocin in Canada during 1996-7. Later on registration has been reached in EU as well, for the treatment and facilitation of protracted delivery labours.
Deamino-carba1-2-O-metyltyrosine-Oxytocin (Carbetocin)
In the group of “vasopressin-like” peptides, the research has focused on the both anti-diuretic and pressoric activities and their selectivity. The extension of the peptide sequence of LVP at the N-terminal part resulted in a compound named Glypressin or Terlipressin.
The very protracted and low pressoric activity of this analogue is currently being used for the treatment of bleeding from gastric and duodenal ulcers and also for the treatment of esophageal varices.
The most successful analogue of Arginin-vasopressin has been prepared by substitutions of L-Arg in position 8 by D-Arginin and at the same time in position 1, where Cysteine was substituted by 3-mercaptopropionic acid (Mpa). In this way, a compound having very high specific antidiuretic and very negligible pressoric activity was obtained:
1-deamino-8-D-arginin-vasopressin (DDAVP) Desmopressin
DDAVP is nowadays broadly used drug for the treatment of diabetes insipidus, enuresis nocturna, Hellebrand disease and other bleeding hemophilic-like disorders. No doubt Desmopressin can be considered as the highlight and blockbuster of the IOCB and in many aspects (including economical) the most successful story of peptide research at the IOCB.
All of the above-mentioned peptide drugs were produced in Czechoslovakia at the Leciva SPOFA pharmaceutical company and after 1989 at Czech subsidiaries of Ferring and Polypeptide Laboratories companies.
It is necessary to note that Desmopressin, Terlipressin and Carbetocin were licensed by the Czechoslovak Academy of Sciences to the Swedish firm Ferring AB in the late sixties. All three compounds are nowadays produced as bulk pharmaceutical chemicals at the Polypeptide Laboratories group mainly in Scandinavia and India in very large several ten-kilo quantities, and Desmopressin is the diamond and flagship among the peptide drugs produced worldwide at present.
Behind the peptide research at the IOCB, there were mainly three excellent peptide chemists, who were carrying out the scientific research and development of the above mentioned peptide drugs and whose names should be mentioned here:
Prof. Josef Rudinger and Dr. Milan Zaoral and Dr. Karel Jost.
All three men were excellent peptide and organic chemists, and for this reason peptide chemistry in our country reached the highest and internationally recognized level during their life and later. Needless to mention, there were many others peptide organic chemists and biochemists at IOCB involved in this interesting and fruitful peptide field. Some of them cooperated strongly to industrial research, which made possible later on the full development of interesting peptide molecules in to the patented active pharmaceutical ingredients.
Most of peptide chemists are also members of the European Peptide Society (EPS). This society organizes European peptide symposium, which is held every two years in a nominated European country. The very first EPS was organized by Prof. Josef Rudinger in Prague in 1958 and tradition of these large meetings is maintained till the present days. European Peptide Society is awarding excellent peptide chemists with the “Rudinger Award “ for lifelong work in the peptide field. Other highly recognized awards given by EPS are “Leonidas Zervas” and “Miclós Bodanszky” awards for the nominated excellent peptide scientists. These chosen scientists then have the privilege to give a very honoured lecture during the EPS symposia.
In the 1950s and early 1960s, the investigation of plant (Compositae family) metabolites made the Department one of the world centers in the area of natural product chemistry with many priorities in the identification and structure determination of natural products. The discovery in chamomile of an anti-inflammatory sesquiterpene, chamazulene, initiated the original synthesis of its derivative, guaiazulene I (1,4-dimethyl-7-isopropylazulene) and finally resulted in the preparation of DERMAZULEN, a commercial ointment with anti-allergic, anti-inflammatory, antipyretic, and antiseptic properties, still available today.
An investigation of insect hormones and hormone analogues, and the search for the potential of their use as an alternative means of insect pest control was another area in which the DNP obtained international recognition. A large array of juvenile hormone analogues (juvenoids) synthesized in the institute has been investigated for their effect on the physiology, development, reproduction and behavior of insects. This extensive research had led to the development of several new technologies that were field-tested and some have been patented and applied. Most notably, a technique based on the juvenoid methoprene against the pharaoh’s ant (Monomorium pharaonis) was designed. A widely-used commercial preparation LAFAREX®, which locally exterminated this troublesome pest, was an outcome of this research.
SEPARATION OF LU-177 FOR MEDICAL APPLICATION
Lutetium-177 (Lu-177) is an important therapeutic radionuclide used to treat cancer. Targeted drugs deliver Lu-177 to tumors where it emits beta particles that destroy the cancer cells. Many types of solid tumors can be treated in this way, and there are numerous drugs in clinical trials that use Lu-177. However, preparing the radioactive Lu-177 for thousands of patients is a significant challenge. It must be perfectly separated from neutron-irradiated ytterbium-176, which is chemically similar. The purity required for medical use is high, and it must be achieved quickly to prevent losses due to radioactive decay. This makes the large-scale separation of Lu-177 extremely difficult.
Dr. Miloslav Polášek, Head of IOCB Prague’s Coordination Chemistry Research Group, has developed a unique technology that is much faster than the current methods for the separation of Lu-177 from Yb-176. The technology is based on molecules called chelators that wrap around the atoms of lutetium or ytterbium. The molecules were specially designed to recognize and amplify the small differences between these two elements. This technique achieves efficient separation in only 10 minutes using a conventional HPLC system, compared to many hours required by other separation technologies.
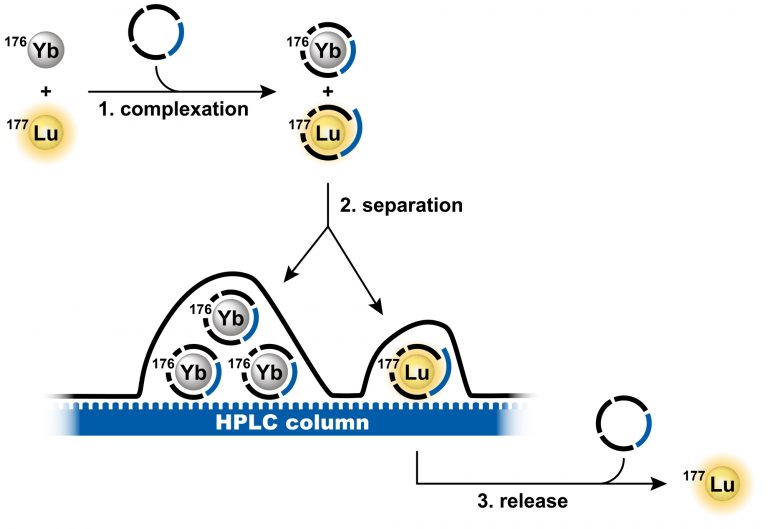
Schematic depiction of the separation method principle. (Credit: Tomáš David).
In May 2019, the Institute of Organic Chemistry and Biochemistry of the CAS (IOCB Prague) signed an agreement providing SHINE Technologies, LLC (SHINE) with a global and exclusive license to this novel separation technology.
In January 2020, SHINE, GE Healthcare and IOCB Prague announced the production of the first Lu-177 doses. The prepared purity and amount demonstrated the suitability of the technology for large-scale Lu-177 production. Most importantly, the lot passed GE Healthcare’s quality control testing, including internationally recognized radionuclide purity standards.
The success story continued in November 2020, when SHINE made the first commercial sales of Lu-177 prepared using the licensed separation technology. SHINE is currently completing a large-scale production facility in Janesville, Wisconsin, with a capacity to make 300,000 doses of Lu-177 per year. We can expect that the demand for Lu-177 will grow rapidly, as more clinical studies demonstrate the therapeutic power of Lu-177.
SHINE and IOCB Prague maintain a strong relationship and collaborative spirit outside of their purely commercial activities. In September 2021, they signed a collaboration agreement to foster further basic research of medical radionuclides by Dr. Polášek’s team at IOCB Prague.
SHINE TECHNOLOGIES
SHINE is a fusion technology company working to become the world’s leading provider of radioisotopes for nuclear medicine in the near term. Since 2019, SHINE has enhanced its ability to fill critical needs in the rapidly growing therapeutic isotope market. Its therapeutics division is currently commercializing the production of Lu-177, a therapeutic isotope, which then needs to be combined with a disease-specific target molecule to treat cancer.
More about Miloslav Polášek Group: https://polasek.group.uochb.cz/en
More about SHINE Technologies: https://www.shinefusion.com/




